For CAR-T therapy immune cells (T-cells) are isolated from the blood, genetically engineered to carry a chimeric antigen receptor (CAR) on the membrane, expanded, and finally administered back into patients’ bloodstream. These CAR-T cells successfully find, bind, and kill the tumor cells.
Clinical trials have shown huge remission rates, of up to 94%, in severe forms of blood cancer. These results and the approval of the first commercially available CAR-T therapy in 2017 have fed the expectations of patients and industry alike.
More than 1000 clinical trials are currently conducted, and the pipeline is strongly growing. Existing manufacturing technologies not only show limitations in quality and scalability but also lead to high therapy costs. Current production concepts need transformation to increase capacity substantially and meet upcoming demand.
Sarcura's Solution

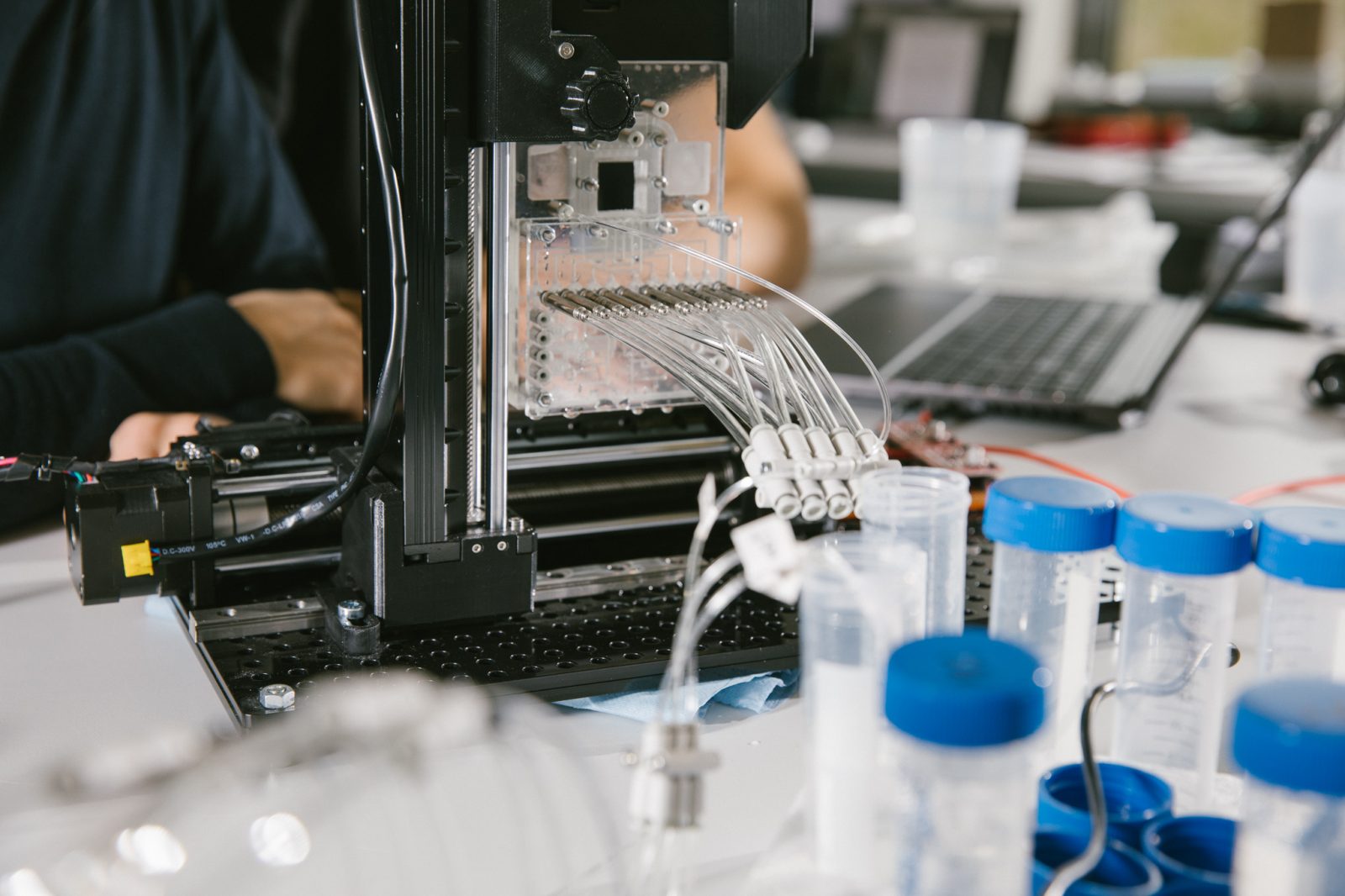
Machine intelligence, controlling robust and reliable automation, is crucial for eliminating human operations and documentation steps. This advancement not only improves process quality but also enables scalability, which is absolutely key to industrializing pharmaceutical cell manufacturing.

Semiconductor technology allows the integration of electronic and photonic structures on silicon substrates.
On-Chip functionality embedded in microfluidic structures enables process management down to the cellular level.

Functional modules, incorporated in a closed single-use cartridge, allow customized process layouts and conceptional flexibility to process different cell types and address individual user needs.
Benefits
Integrated process and quality control on the cellular level provide real-time process information
Closed automated system with integrated process and quality control reduce operating and infrastructure time and cost
Industrial automation and the elimination of manual interaction enable scalability and increase output
Data-based control regimes ensure reliability and reproducibility